The Last Breath of the Mammoths

It is sometimes said that mammoths are the most familiar of the extinct. Unlike dinosaurs, whose bones must be fleshed out by imagination, mammoths left us their bodies: tusks, ivory , skin preserved in permafrost, even stomachs still packed with the grasses of the Ice Age steppe. They are written into our stories, painted on Paleolithic cave walls, carved into tools and ornaments, invoked in myths. And unlike dinosaurs, mammoths did not vanish in the distant reaches of prehistory. When the Great Pyramid of Giza was already standing, small herds of mammoths still walked the windswept tundra of Wrangel Island in the Siberian Arctic. In a very real sense, they are both gone and just barely gone.
Today, the mammoth story is being rewritten not just from bones and tusks, but from fragments of DNA. Genomics has stretched the reach of paleontology from the level of visible morphology to the invisible code of life, peeling back layers of evolutionary history that had seemed forever out of reach. That story begins, like so many Ice Age tales, in Africa.
Origins: From African Beginnings to Global Wanderers
The mammoth lineage (Mammuthus) arose in Africa about five million years ago (Lister et al., 2005; Werdelin and Sanders, 2010). Their ancestors belonged to the broader family of elephants (proboscideans), which had already been experimenting with body forms ranging from squat, hippo-like species to the towering straight-tusked elephants of Eurasia. From that African origin, mammoths spread northward into Eurasia and, eventually, into the Americas.
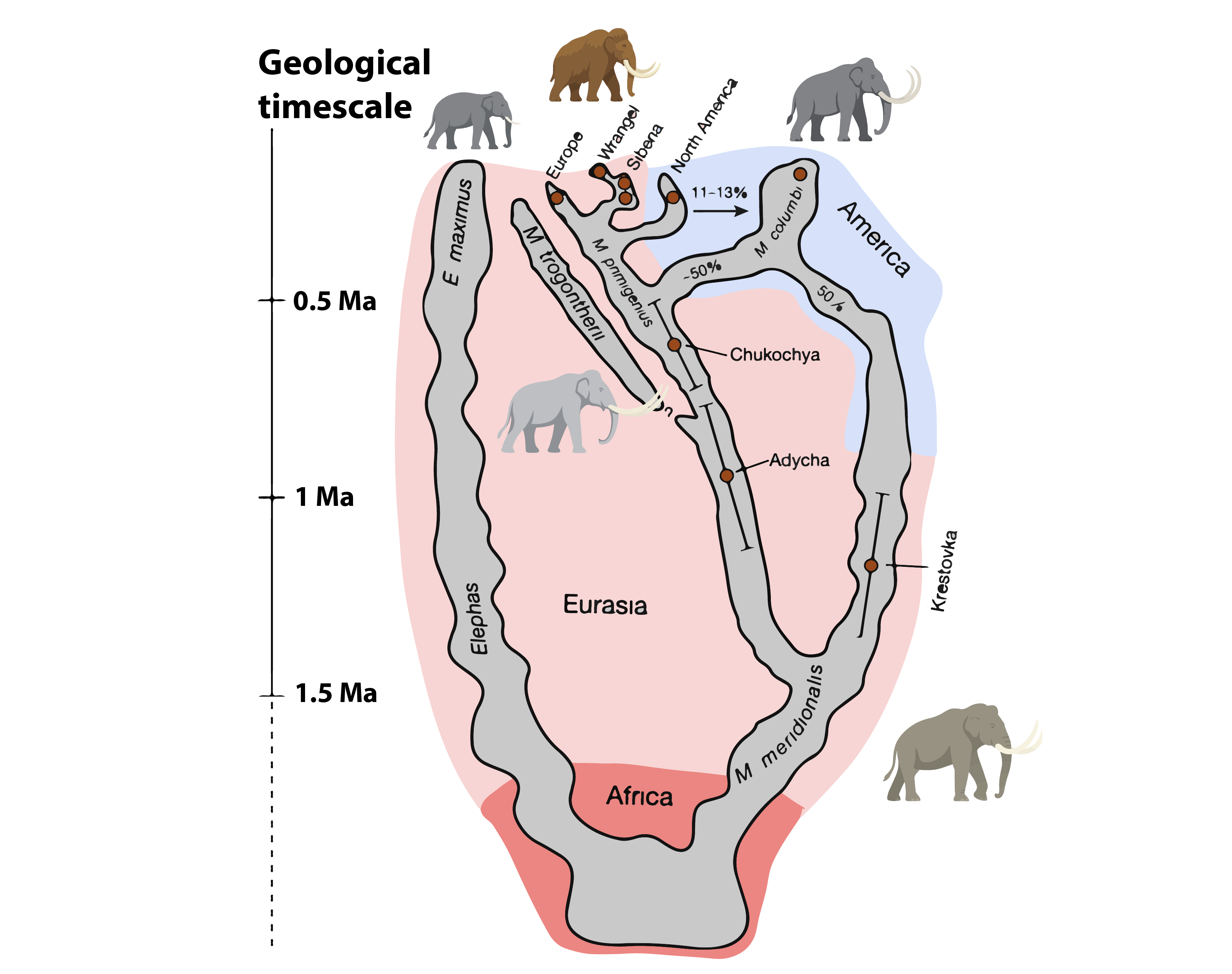
The first recognizably “mammoth-like” species was the southern mammoth (Mammuthus meridionalis), which reached Europe about 2 million years ago. These were giants with relatively simple molars, adapted to mixed woodland and grassland environments. Around 1.7 million years ago, a descendant lineage produced the steppe mammoth (Mammuthus trogontherii), an animal of truly continental range from Western Europe to East Asia. With taller, more enamel-rich molars suited to grinding coarse grasses, M. trogontherii embodied the open steppe lifestyle.
From this branching point, evolution produced two of the most iconic mammoth species: the woolly mammoth (M. primigenius), adapted to the high Arctic, and the Columbian mammoth (M. columbi), which roamed the temperate grasslands of North America (Lister and Sher, 2015). Recent genomic breakthroughs reveal that the Columbian mammoth was not a simple descendant of steppe mammoths, but a hybrid species. Indeed, around 1.5 million years ago, a previously unknown lineage : the so-called “Krestovka mammoth” crossed into North America. Later, perhaps around 400,000 years ago, woolly mammoths from Siberia interbred with those North American inhabitants. The Columbian mammoth’s genome today reads as a mosaic: roughly half woolly, half Krestovka (van der Valk et al., 2021). In other words, a hybrid speciation event produced the largest proboscidean to ever graze the plains of North America.
These evolutionary braids remind us that the tree of life is often less a tree and more a braided river: lineages diverge, reconnect, and leave behind genomes full of ghostly admixture.
Mammoths and Humans: An Ancient Relationship

By the time anatomically modern humans began spreading across Eurasia, mammoths were already established fixtures of Ice Age ecosystems. For hunter-gatherers, they were not only prey but also providers of shelter and raw material. In the cold steppes of Ukraine and Russia, humans built houses from mammoth bones, ribcages forming walls, tusks arching as doorways. In Chauvet and Rouffignac caves, mammoths stride across limestone walls in ochre and charcoal. To our Upper Paleolithic ancestors, mammoths were neighbors and symbols as much as food sources.
The deep bond between mammoths and humans is a double-edged legacy. Our ancestors revered them, depended on them, and in the end may have helped usher them toward extinction in certain part of the world.
Built for the Ice Age
If mammoths were to thrive in the frigid mammoth steppe, the mosaic of grasses, herbs, and tundra that stretched from Spain to Alaska, they needed more than size and tusks. They needed molecular innovations.
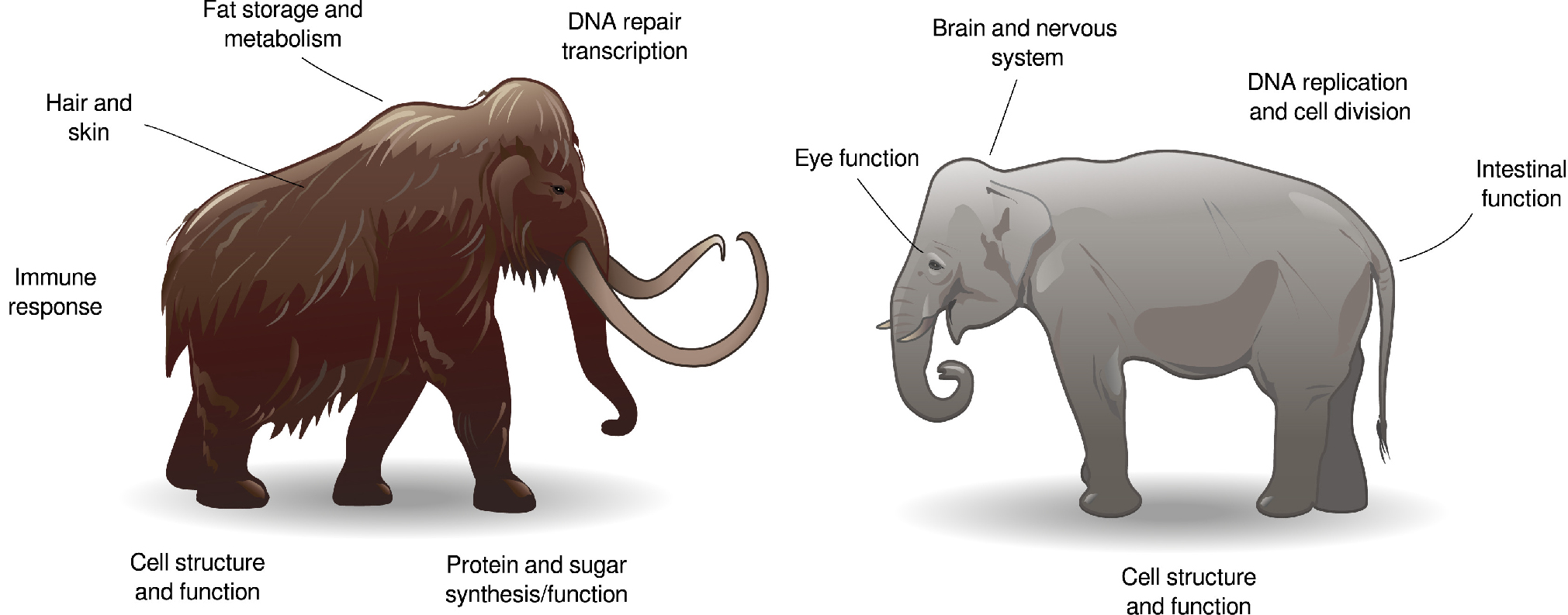
Genomic research has revealed just how profound woolly mammoth adaptations were. Hemoglobin genes carried amino acid substitutions that improved oxygen release in the cold (Campbell et al., 2010). Their genomes also contained modifications in genes linked to hair growth, skin structure, fat metabolism, and temperature sensation, pointing to thick insulating coats, sebaceous glands producing protective oils, substantial fat layers, and altered cold perception (Lynch et al., 2015). Many of these features were already present at the origin of the species, with subsequent evolution further shaping traits such as ear size, body proportions, and skeletal form (Díez-del-Molino et al., 2023). Compared to their elephant relatives, woolly mammoths thus displayed shorter ears and tails to reduce heat loss, compact barrel-shaped bodies, and dense insulation every aspect of their form attuned to survival in a bitter, windswept, ice-dominated world.
Ancient DNA: Reading Ghosts in Molecules
How do we know all this? Through the revolution of ancient DNA.
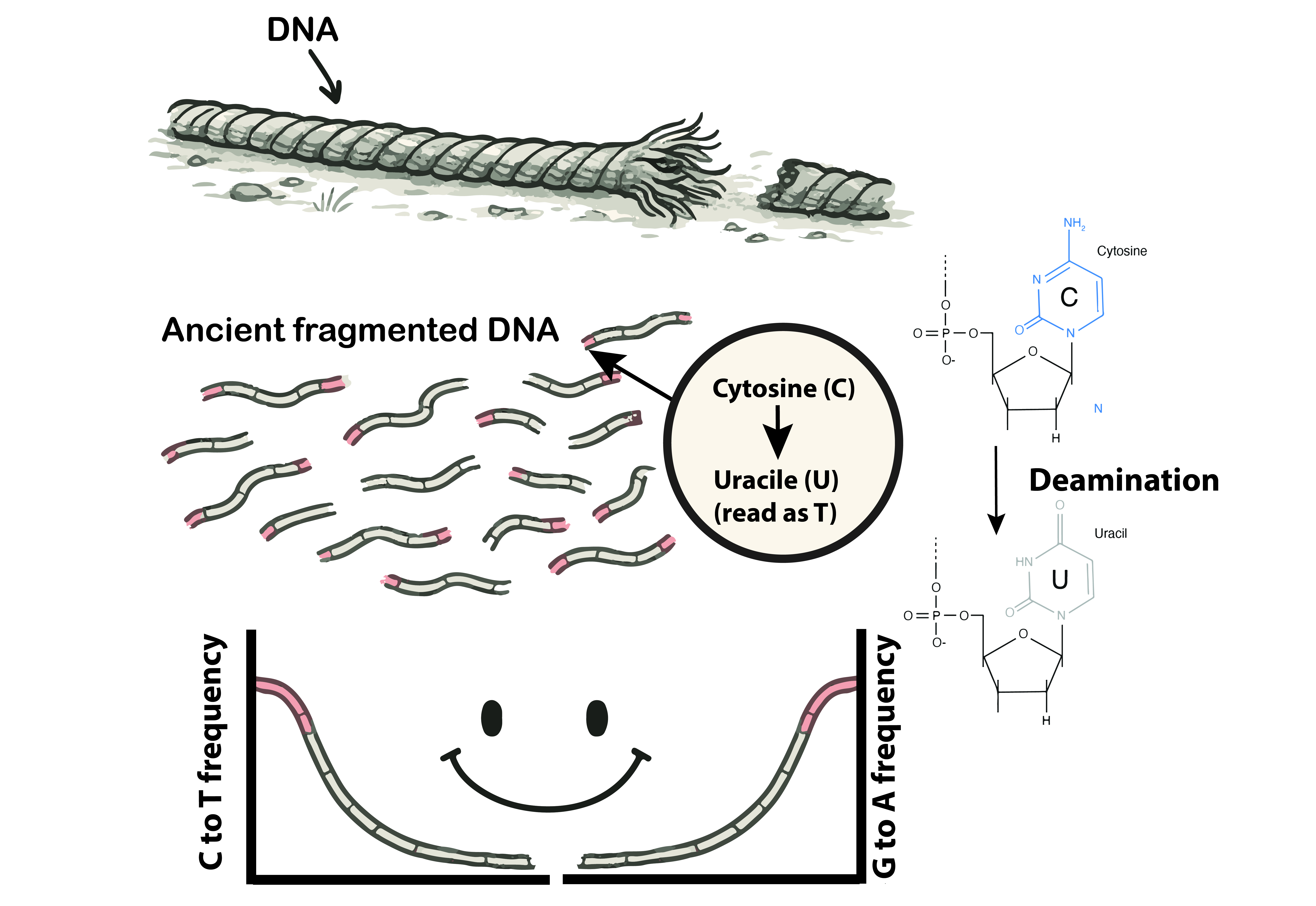
DNA is a fragile molecule. After death, it breaks down first into fragments, then into chemical damage. Imagine a rope left in the elements: the ends fray first, while the middle remains intact. Ancient DNA fragments are like that rope, often just 30–70 base pairs long, with characteristic damage at their ends. One telltale signature is cytosine deamination, where cytosine bases spontaneously mutate into uracil, read as thymine during sequencing. When graphed across DNA fragments, these changes create a U-shaped pattern we call “smiley plot” that authenticates the DNA as ancient rather than modern contamination (Briggs et al., 2010).
For decades, it was thought impossible to recover DNA older than 100,000 years. The record slowly crept backward: Neanderthals, Denisovans, cave bears. Then a horse genome, nearly 700,000 years old, shattered expectations (Orlando et al., 2013). Finally, in 2021, researchers announced the recovery of mammoth DNA more than one million years old from molars preserved in Siberian permafrost (van der Valk et al., 2021). That study pushed paleogenomics into “deep time,” showing that DNA can persist orders of magnitude longer than once imagined.
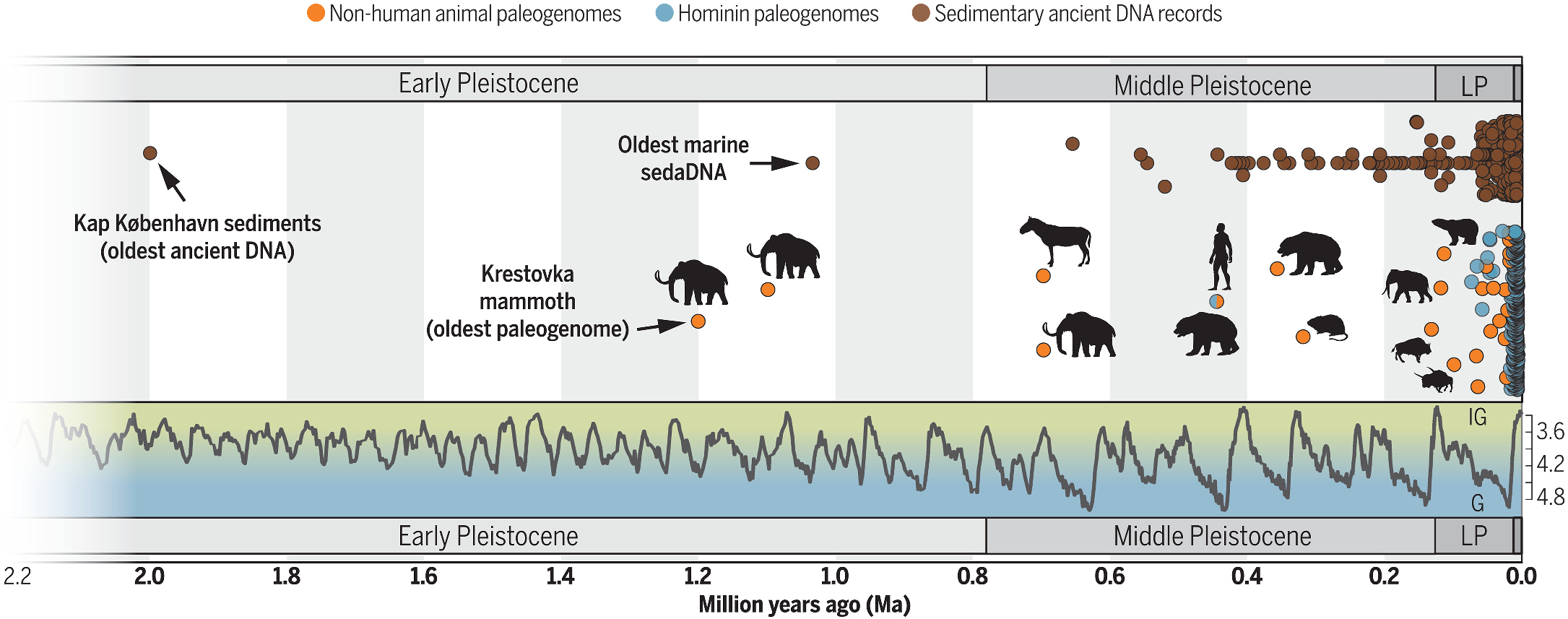
The oldest ancient DNA was retrieved from sediments in Greenland (nearly two million years old) includes fragments from mastodons, reindeer, and plants, hinting at entire lost ecosystems recoverable from soil (Kjaer et al., 2022). But mammoths still hold the crown for the oldest host-associated genomes: full nuclear data from individual animals, stretching the genetic record back into the Early Pleistocene.
The Last Stronghold: Wrangel Island
For most of their history, mammoths were continental wanderers. But their final chapter played out in isolation.
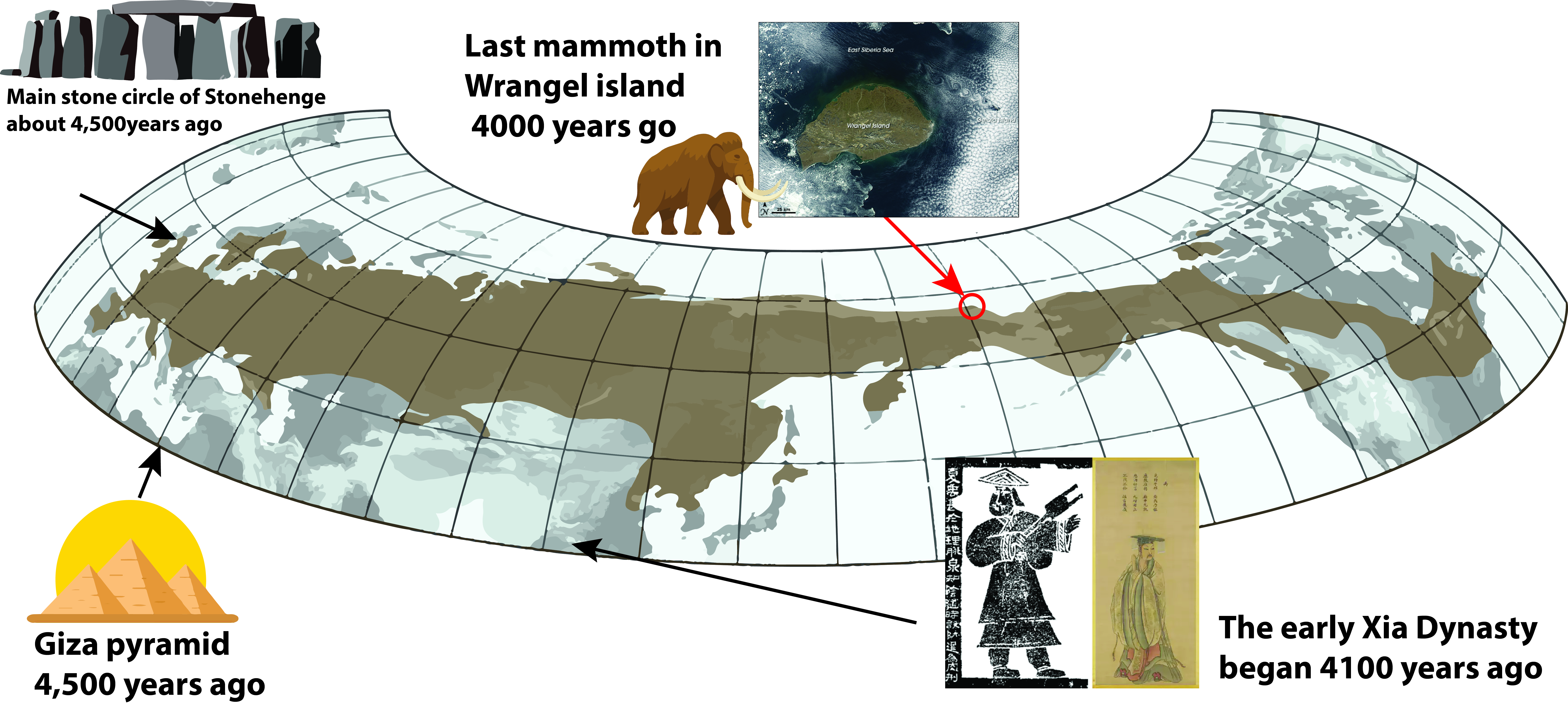
As the Ice Age ended about 11,000 years ago, mammoths vanished from mainland Eurasia and North America, victims of shifting climates and hunting pressure. Yet small relict populations held on on St. Paul Island in Alaska until about 5,600 years ago, and most famously on Wrangel Island 4000 years ago, a desolate Arctic refuge north of Siberia. There, a tiny founding group perhaps as few as eight individuals managed to survive long after mammoths disappeared elsewhere (Palkopoulou et al., 2015).
At their peak, Wrangel’s mammoths numbered about 200–300 individuals, though genetic simulations suggest the population may have dipped as low as just eight during the initial Holocene bottleneck. Such small populations face genetic perils: loss of diversity, inbreeding, and accumulation of harmful mutations, a process biologists call “mutational meltdown.” Earlier work suggested that Wrangel mammoths were spiraling toward such a fate, with degraded olfactory genes, defective immune genes, and pseudogenized skin proteins (Rogers and Slatkin, 2017).
However, new genomic analyses paint a more nuanced picture (Dehasque et al 2024). The Wrangel population recovered rapidly after its bottleneck and then remained demographically stable for nearly 6,000 years. Rather than collapsing under mutational load, the population appears to have purged many highly deleterious mutations, though moderate-impact ones continued to accumulate. Crucially, there is no evidence of a long-term demographic decline prior to their disappearance. Instead, their sudden extinction around 4,000 years ago may have been triggered by an external shock such as a disease outbreak, abrupt environmental change, or both acting on an already genetically compromised population.
Wrangel mammoths disappeared about 4,000 years ago a time when humans in Egypt were already inscribing hieroglyphs and building pyramids. In Britain, the main stone circle of Stonehenge had already been standing for centuries (constructed ~2500 BCE, ~4,524 years ago). In China, the early Xia Dynasty was beginning (~2070 BCE, ~4,094 years ago), considered the first dynasty in legend and history. Crucially, archaeological evidence suggests humans did not reach Wrangel until after the mammoths were gone (Arppe et al., 2019). Unlike the mainland extinctions, where hunting likely played a role, Wrangel’s mammoths seem to have succumbed to the combined weight of genetic fragility and environmental change.
The Invisible Passengers: Mammoths and Their Microbes
For decades, scientists have been sequencing mammoth DNA to reconstruct the lives of these Ice Age giants, tracing their evolution, migrations, and eventual decline. Yet one crucial dimension of their biology long remained hidden: the microbes that lived alongside them. Just as modern elephants harbor a complex community of bacteria and other microorganisms that shape their health, mammoths, too, carried a microscopic entourage.
Recovering those ancient traces is anything but simple. Extracting mammoth DNA from a bone is already like searching for a needle in a haystack; isolating microbial DNA that once lived alongside the mammoth is closer to looking for dust on the needle itself. After death, the mammoth’s body quickly became a magnet for foreign DNA: soil microbes infiltrated its tissues, decomposition bacteria flourished, and modern contaminants were introduced during excavation and handling. These outsiders vastly outnumbered the authentic microbes that had truly inhabited the animal (Duchêne et al. 2020). Even the genuine remnants were badly degraded—ancient DNA tends to be fragmented, chemically damaged, and buried in background noise. The challenge was even steeper for microbial genomes, which were already minuscule compared with the mammoth’s own.
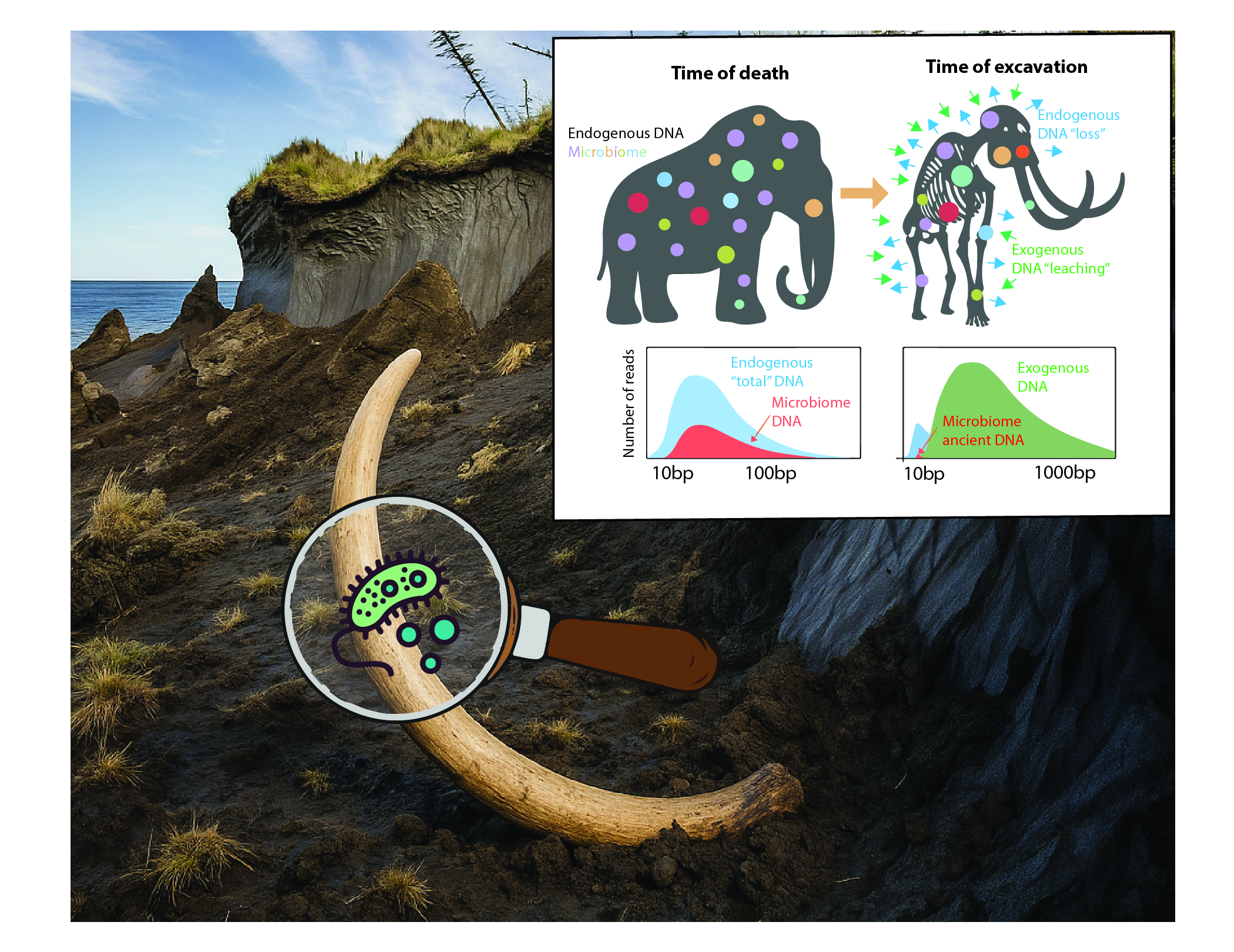
Despite these obstacles, we recently managed to recover ancient microbial DNA from mammoth remains, opening a new frontier in the study of microbes found in extinct animals. The scale of the dataset was unprecedented. We screened DNA from 483 mammoth specimens, not only woolly mammoths but also much older steppe mammoths, covering more than a million years of evolutionary history. Therefore, from the tusk, tooth or skin of these specimens, we were able to identify six groups of bacteria that appear to have been genuine host-associated companions, persisting with mammoths across vast stretches of time and space.
Among the most remarkable discoveries was a partial genome of Erysipelothrix from a 1.1-million-year-old steppe mammoth which appear to be the oldest authenticated host-associated bacterial DNA ever recovered. Some of the microbes we found carry sobering implications. For example, we detected strains closely related to a bacterium that in 2020 caused fatal septicemia in African elephants. This does not mean the mammoth we studied died from such an infection, but it suggests that, like their modern cousins, mammoths may have been susceptible to this pathogen under stressful conditions. Mammoths also harbored familiar oral microbes such as Streptococcus, bacteria that in many animals can contribute to dental disease or another oral bacteria that was probably transferred through mammoth lineage for thousands of years.
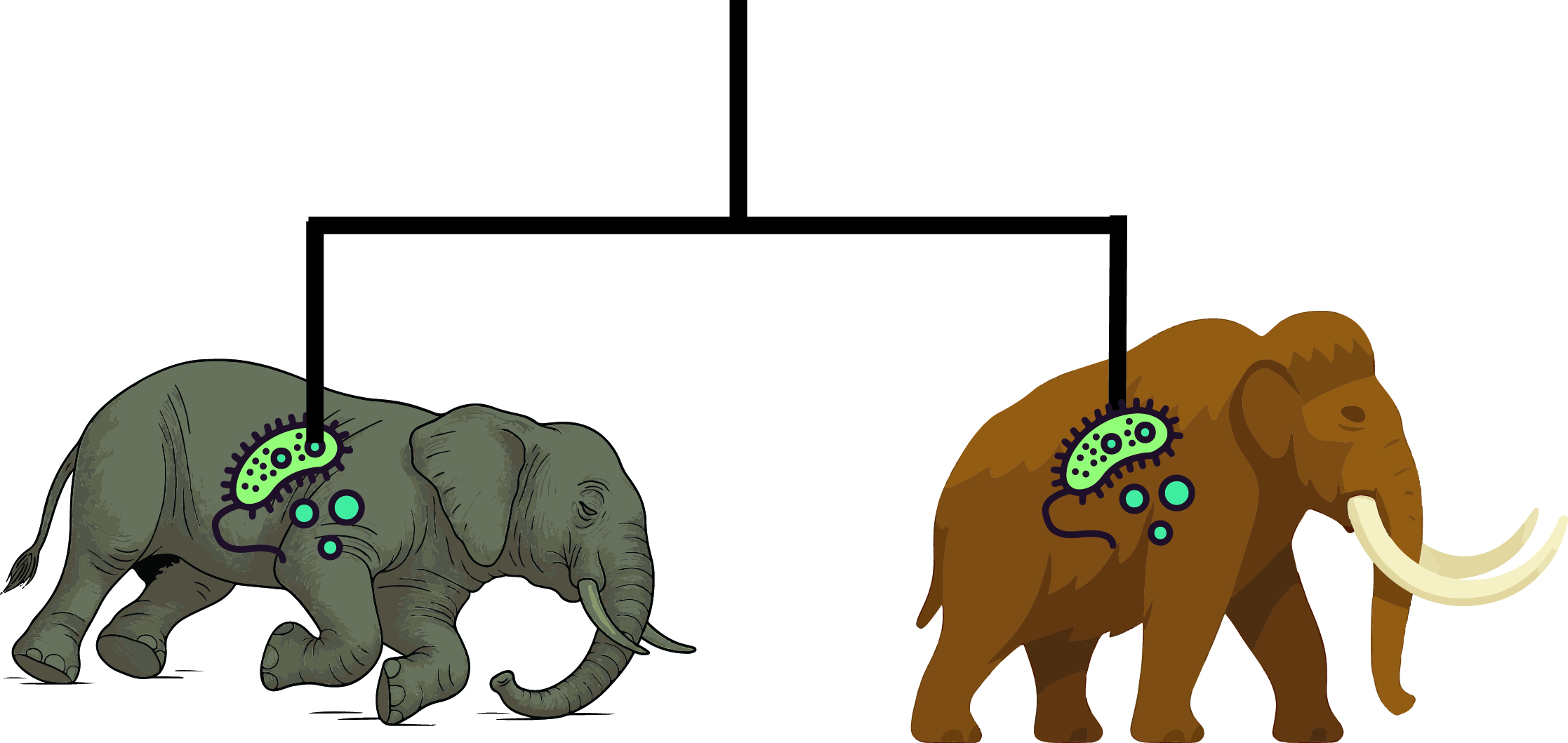
The picture that emerges is not one of microbes driving mammoth extinction, but of microbes as constant, if invisible, companions in their evolutionary journey. Some may have been harmful, others harmless passengers, and some perhaps even beneficial, playing roles in digestion and survival. Together, they formed an unseen but enduring part of what it meant to be a mammoth.
This work is only the begainning. Future research will expand our understanding of the other microbes that shared the mammoth’s world including viruses. So far, no viral genomes have been isolated, but one day we may detect ancient relatives of pathogens that today devastate elephants, such as elephant endotheliotropic herpesvirus. Each step brings us closer to seeing mammoths not just as giant Ice Age icons, but as whole ecosystems animals whose lives and health were deeply entwined with a hidden microbial world that endured for over a million years.
Conclusion
Mammoths remind us that extinction is rarely a single event. It is a drawn-out process, braided from climate, ecology, demography, and biology. Thanks to ancient DNA, we can now listen in on that process at an unprecedented molecular resolution. We can see how mammoths adapted to ice and cold, how their lineages braided into hybrids, how their genomes faltered in isolation, and even how their microbes marched alongside them through deep time.
The dialogue between genes, climate, bodies, and microbes is not just about the past. It resonates with the present, as elephants the mammoths’ surviving cousins face pressures from habitat loss, poaching, and disease. The mammoth’s long shadow is not only a story of what was lost, but also a warning of what might yet be lost again.
References
Arppe, L., Ukkonen, P., Lister, A. M., Mannermaa, K., & Zazula, G. D. (2019). Wrangel Island mammoths and the chronology of their extinction. Quaternary Science Reviews, 209, 57–64. https://doi.org/10.1016/j.quascirev.2019.02.011 Briggs, A. W., Stenzel, U., Johnson, P. L. F., Green, R. E., Kelso, J., Prüfer, K., Meyer, M., Krause, J., Ronan, M. T., Lachmann, M., & Pääbo, S. (2010). Patterns of damage in genomic DNA sequences from a Neandertal. Proceedings of the National Academy of Sciences, 107(36), 16131–16136. https://doi.org/10.1073/pnas.1004946107
Campbell, K. L., Roberts, J. E. E., Watson, L. N., Stetefeld, J., Sloan, A. M., Signore, A. V., Howatt, J. W., Tame, J. R. H., Rohland, N., Shen, T. J., et al. (2010). Substitutions in woolly mammoth hemoglobin confer biochemical properties adaptive for cold tolerance. Nature Genetics, 42, 536–540. https://doi.org/10.1038/ng.574
Díez-del-Molino, D., Dehasque, M., Chacón-Duque, J. C., Heintzman, P. D., van der Valk, T., Dalén, L., et al. (2023). Genomics of adaptive evolution in the woolly mammoth. Current Biology, Published online April 7, 2023. https://doi.org/10.1016/j.cub.2023.03.084
Guinet, B., Oskolkov, N., Moreland, K., Nikolskiy, P., Dalén, L., van der Valk, T., et al. (2025). Ancient host-associated microbes obtained from mammoth remains. Cell, Published online September 2, 2025. https://doi.org/10.1016/j.cell.2025.08.003
Kjaer, K. H., et al. (2022). A 2-million-year-old ecosystem in Greenland uncovered by environmental DNA. Science, 379(6629), 107–111. https://doi.org/10.1126/science.abm3916
Lister, A. M., Sher, A. V., van Essen, H., & Wei, G. (2005). The pattern and process of mammoth evolution in Eurasia. Quaternary International, 126–128, 49–64. https://doi.org/10.1016/j.quaint.2004.04.014
Lister, A. M., & Sher, A. V. (2015). Evolution and dispersal of mammoths across the Northern Hemisphere. Science, 350, 805–809. https://doi.org/10.1126/science.aac5660
Lynch, V. J., Bedoya-Reina, O. C., Ratan, A., Sulak, M., Drautz-Moses, D. I., Perry, G. H., Miller, W., & Schuster, S. C. (2015). Elephantid genomes reveal the molecular bases of woolly mammoth adaptations to the Arctic. Cell Reports, 12(2), 217–228. https://doi.org/10.1016/j.celrep.2015.06.027
Orlando, L., Ginolhac, A., Zhang, G., Froese, D., Albrechtsen, A., Stiller, M., Schubert, M., Cappellini, E., Petersen, B., Moltke, I., et al. (2013). Recalibrating Equus evolution using the genome sequence of an early Middle Pleistocene horse. Nature, 499, 74–78. https://doi.org/10.1038/nature12323
Palkopoulou, E., Mallick, S., Skoglund, P., Enk, J., Rohland, N., Li, H., Omrak, A., Vartanyan, S., Poinar, H., Götherström, A., Reich, D., & Dalén, L. (2015). Complete genomes reveal signatures of demographic and genetic declines in the woolly mammoth. Current Biology, 25, 1395–1400. https://doi.org/10.1016/j.cub.2015.04.007
Rogers, R. L., & Slatkin, M. (2017). Excess of genomic defects in a woolly mammoth on Wrangel island. PLoS Genetics, 13(3), e1006601. https://doi.org/10.1371/journal.pgen.1006601
van der Valk, T., Pečnerová, P., Díez-del-Molino, D., Bergström, A., Oppenheimer, J., Hartmann, S., Xenikoudakis, G., Thomas, J. A., Dehasque, M., Sağlıcan, E., et al. (2021). Million-year-old DNA sheds light on the genomic history of mammoths. Nature, 591, 265–269. https://doi.org/10.1038/s41586-021-03224-9
Werdelin, L., & Sanders, W. J. (Eds.). (2010). Cenozoic Mammals of Africa. University of California Press.
Enjoy Reading This Article?
Here are some more articles you might like to read next: