Lessons from Rinderpest to eradicate Measles?
Introduction
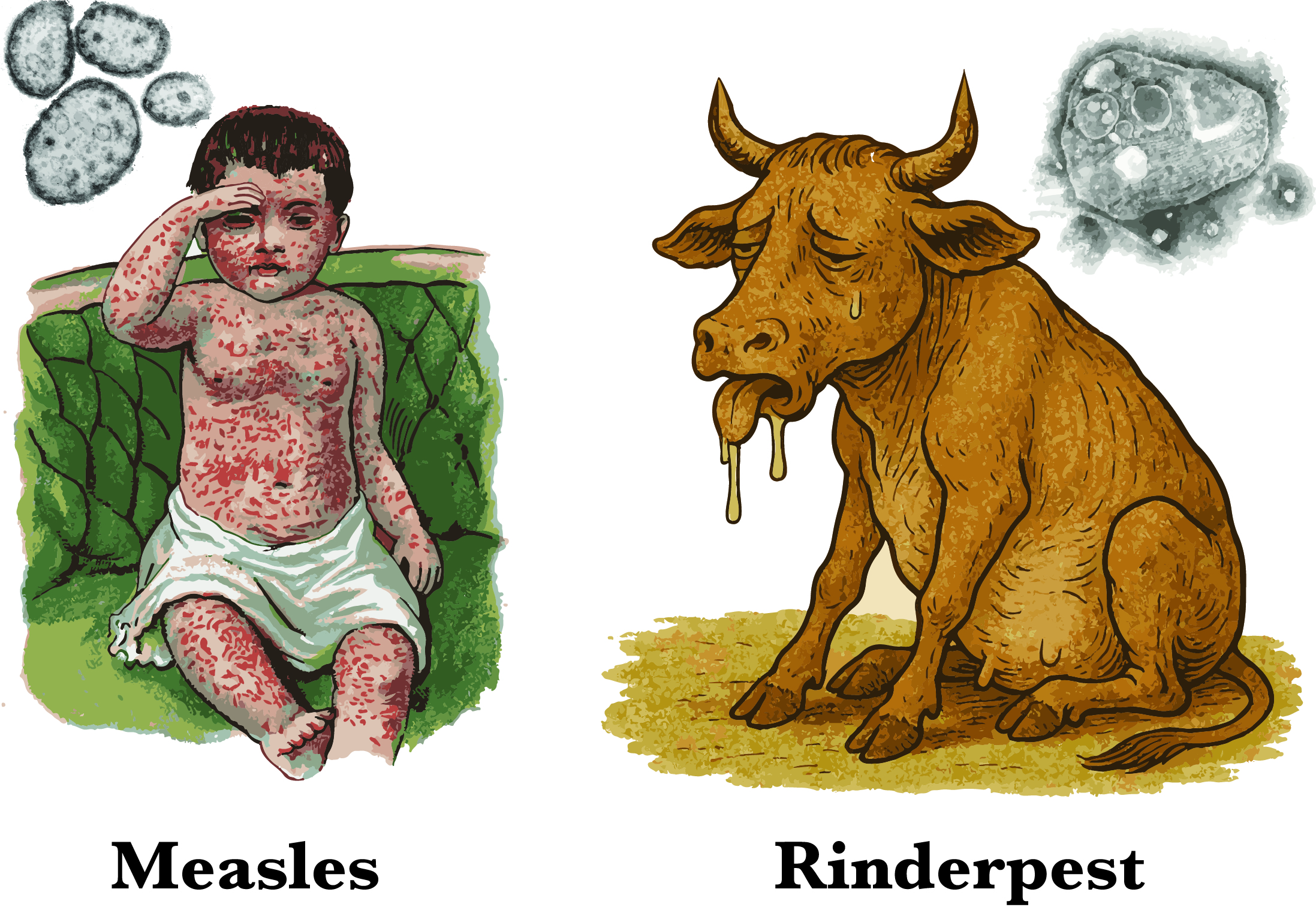
Measles and rinderpest are two viral diseases that have left a profound mark on history : one through its devastating toll on children’s health, which persists worldwide today, and the other through massive economic losses inflicted on livestock farming across many regions. At first glance, these viruses may seem entirely distinct, yet they surprisingly share a relatively recent common ancestor.
Around 3,000 years ago, an ancestor of the rinderpest virus made the leap across species and adapted to humans, eventually evolving into the measles virus. Since that pivotal moment, each virus has followed its own evolutionary path: measles with human populations, and rinderpest with livestock.
In this article, we’ll delve into their intertwined origins, trace the separate journeys these viruses have taken, and examine how humanity has succeeded in eradicating one, while the other continues to pose significant challenges today. Ultimately, we’ll explore how the lessons learned from defeating one viral foe might hold the key to overcoming the other.
Rinderpest

Let’s start with a virus that was once feared in the agricultural world: the rinderpest virus. This virus was infecting cattle but could also infect more than 40 other species of artiodactyls (even-toed ungulates)(Morens et al. 2011). Rinderpest was one of the most devastating animal diseases in history. In some epidemics, it decimated up to 90–100% of infected herds, causing millions of cattle deaths worldwide. Its extreme virulence made it a real health and economic disaster. The disease was transmitted through close contact between animals, via respiratory, oral or faecal secretions. After an incubation period of 8 to 11 days, symptoms appeared: fever, mouth ulcers, followed by severe and often fatal diarrhea.
In the 18th century, Europe was hit hard. With no vaccine available at the time, drastic measures were implemented: quarantines, isolation, mass slaughter and disinfection. Thanks to these efforts, the disease was finally eradicated from Europe at the end of the 19th century (Vicq-d’Azyr, 1776).
But elsewhere, rinderpest continued to wreak havoc. In Africa, it triggered a catastrophic pandemic, killing millions of animals, causing a major economic crisis, and exacerbating poverty, malnutrition, and human disease (Morens 2003; Wilkinson 1984)
A global victory thanks to the vaccine

It was not until the 1920s that the first attenuated vaccine was developed, and then the 1950s before a more effective vaccine enabled mass vaccination campaigns, thanks in particular to a coordinated programme between African countries: Joint Programme-15. As a reminder, a vaccine trains our immune system to recognise a pathogen without causing disease. It contains an attenuated or inactivated form of the microbe, or an antigen (often a protein). As a result, our body learns to defend itself. And if the pathogen appears later, the immune system reacts quickly and neutralises the infection.
After the programme’s success until the 1970s, the premature cessation of vaccination led to a resurgence of the virus in Africa, Asia and the Middle East. The WHO responded with several global campaigns (PARC, PACE, GREP, etc.). After decades of international cooperation, the last reported outbreak of rinderpest occurred in Kenya in 2001; however, a 10-year period of active surveillance was necessary before global eradication could be declared in 2011, making it the second virus to be eradicated in history, after smallpox (1980). Today, almost all of the samples still present in laboratories, if any remain, are nothing more than pathological remnants, preserved for research or scientific memory purposes, but having lost all infectious capacity (Butler 2019).
Ultimately, rinderpest not only devastated livestock, it also played an important role in the early history of infectious disease science. It helped inspire one of the earliest theories on the spread of disease (Morens 2003) and even led to the development of a vaccination plan (Anderson and McKay 1994). Scientists studying rinderpest were also the first to demonstrate that mothers could pass on their immunity to their offspring (Huygelen 1997). The global alarm raised by its ravages ultimately led to the creation of the World Organisation for Animal Health (OIE) in 1924.
Measles: a human cousin of rinderpest :

Let us now turn our attention to measles. Like its cousin rinderpest, it is an RNA virus of the Morbillivirus genus, meaning it has a very high mutation rate, giving it the ability to rapidly explore the space of possibilities in order to adapt to its host. However, we will see that in the case of rinderpest and measles, although they mutate rapidly, they tend to remain relatively stable over time.
As we saw above, measles is actually the result of a species jump. In 2020, researchers traced the history of the measles virus from ancient samples (including the lung of a child who died in 1912) (Düx et al. 2020). Their study shows that around 2,000 to 3,000 years ago (roughly when Buddha was living in South Asia and shortly before the birth of Socrates), a bovine virus accidentally found its way into the bodies of unsuspecting humans and then split into two strains: one became measles in humans, the other became rinderpest in cattle.
But why did this species jump occur at precisely this time, and why did it end up establishing itself permanently in humans?
Human density becomes a condition for a virus to jump
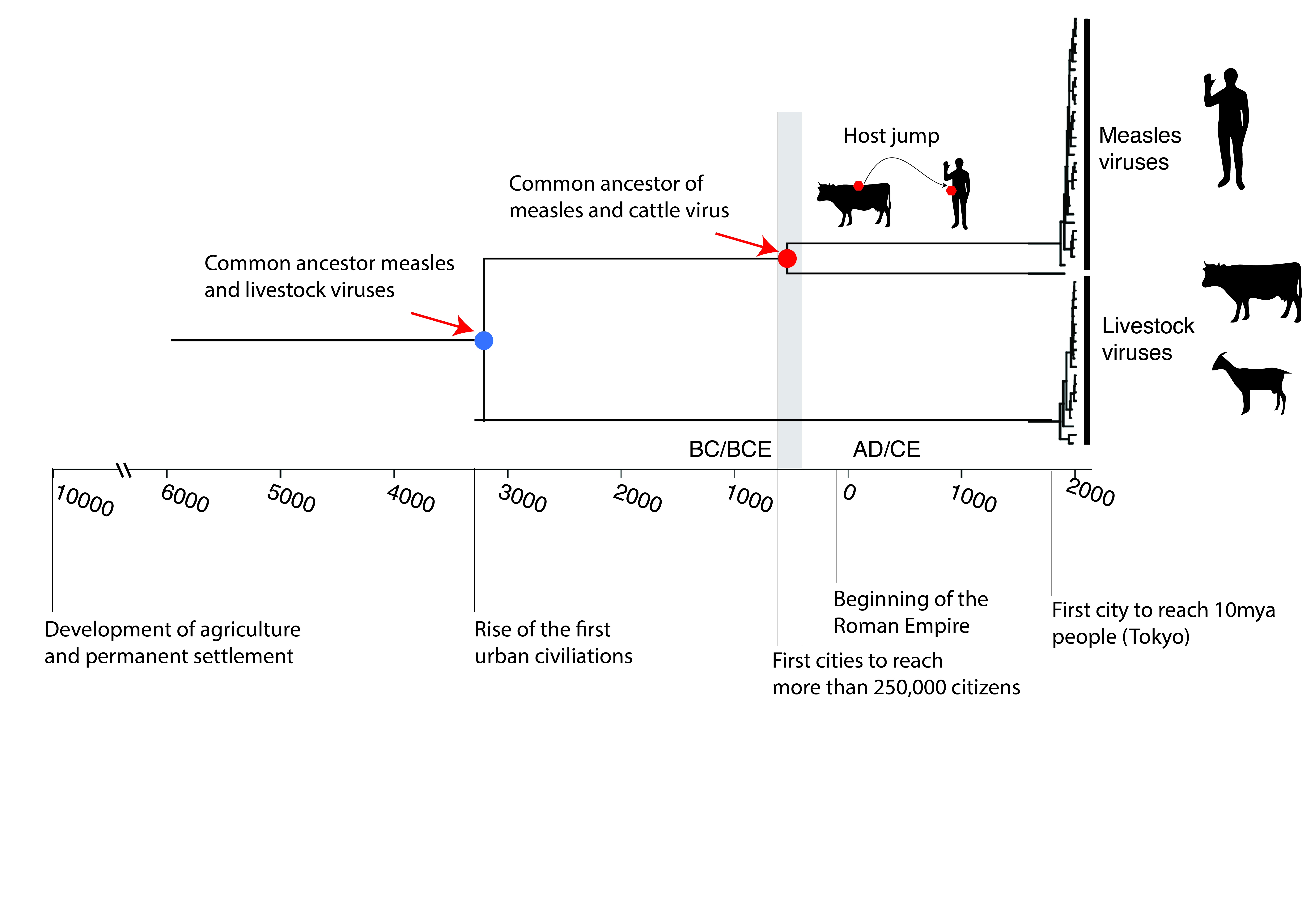
For a virus such as measles to survive long-term in a human population, one essential condition must be met: population density. Measles is an extremely contagious virus that confers lifelong immunity to those who have contracted it. This means that in order to continue circulating, the virus constantly needs new susceptible individuals to infect, particularly children who have never been exposed to it before.
However, in Neolithic, Bronze Age and even early Iron Age human societies, human groups were still too small and too scattered. Villages were modest, towns were embryonic, and exchanges between communities were limited (Brozio et al. 2024). Even if an ancestral measles virus had crossed the species barrier at that time, it would have hit a wall: without new victims, it would have quickly disappeared. Its rapid transmission, combined with the lasting immunity it confers, prevents its survival in small populations.
It was only at the end of the first millennium BCE that conditions changed decisively. Thanks to agricultural, commercial and political advances, large cities capable of housing between 250,000 and 500,000 inhabitants began to appear in certain regions of the world, notably in South Asia, China, the Near East, North Africa and Europe (Clark 2014; Inoue et al. 2015). This threshold is crucial: it represents the critical mass necessary for measles to be transmitted continuously, from one generation to the next, without dying out (Düx et al. 2020).
From that point on, one of these zoonotic jumps, a bovine virus infecting a human, found a favourable environment. In these larger, interconnected societies, the virus was able to maintain itself, evolve and adapt to its new host. It then became a fully-fledged human virus, giving rise to the measles we know today, an endemic virus deeply linked to the history of great civilisations.
Why has measles not yet been eradicated?
Unlike rinderpest, measles still exists. And yet, the current vaccine (MMRV), which has been in use since the 1960s, remains extremely effective (Bankamp et al. 2011; Zemella et al. 2024). In fact, despite the fact that the measles virus is an RNA virus and therefore, in theory, subject to a high mutation rate, it exhibits remarkable antigenic stability. This paradox can be explained by a key biological phenomenon: the measles virus is antigenically monotypic. In other words, the virus’s surface proteins, particularly haemagglutinin (H), which is the main target of the neutralising immune response, are extremely conserved over time and across the various circulating strains (Fulton et al. 2015). This antigenic stability can be explained in part by strong structural and functional constraints: the virus’s surface proteins cannot vary greatly without compromising their ability to interact effectively with the host’s cell receptors. In other words, the virus cannot ‘afford’ to change too much without losing its ability to infect. This contrasts sharply with viruses such as influenza, whose surface proteins evolve rapidly under immune pressure, requiring annual vaccine reformulation.
When an individual naturally contracts measles and survives, they generally acquire lifelong immunity. This means that it is highly unlikely that they will be reinfected later in life, and no additional doses of vaccine are necessary. However, contracting the desease without vaccination is risky for a child. Durthermore, researchers have recently shown that measles infection can cause a form of ‘memory loss’ in the immune system, reducing its ability to remember pathogens it has encountered before. This phenomenon has not been observed in vaccinated children, which further highlights the importance of vaccination in protecting children from severe infection and preventing them from losing previously acquired immunity (Mina et al., 2019). Two doses of the vaccine are recommended to ensure immunity and prevent epidemics, as not all children develop immunity after the first dose.
If vaccination is so effective, why are we failing to eradicate the measle virus?
The answer can be summed up in one word: human resources.
Unlike cattle, which cannot refuse an injection, human beings have that choice. However, this free will can be influenced by many factors: lack of information, mistrust of science, unequal access to healthcare, political tensions, etc. These issues have been exacerbated by events related to the COVID-19 pandemic, which have contributed to increased scepticism and mistrust of vaccines among some members of the public (Lazarus et al. 2023).
This crisis of confidence has worrying consequences, including a reversal of progress in public health. In 2019, for example, the United States, where measles had been officially eliminated since 2000, experienced a resurgence of outbreaks in certain communities with low vaccination rates. For example, the proportion of children who received a first dose of measles vaccine was 83% in 2023, well below the 86% level reached in 2019.
The CDC sounded the alarm:
«The current increase in measles cases is deeply concerning. The United States is once again at risk of losing its measles elimination status due to declining vaccination coverage in certain communities.» (CDC, 2019)
Peter Hotez, a public health expert, summed up the situation as follows:
«Measles is the canary in the coal mine for vaccine-preventable diseases. When it resurges, it’s a sign of broader systemic failure in public health infrastructure and trust.» (Hotez, 2021)
The issue goes beyond medicine
Measles is a good example of how fighting a virus is not just a matter of vaccination, but also of communication, social justice, logistics and trust in science. Even with an excellent vaccine, a lack of coordination or will can ruin all efforts.
Even today, despite massive campaigns by the WHO, Gavi and UNICEF, more than 107,000 children died of measles in 2023, a completely preventable disease that has already saved the lives of nearly 60 million people between 2000 and 2023.
In conclusion: We should learn from the past and protect the future. The eradication of rinderpest, although little known to the general public, is a major victory for science, cooperation and international solidarity. It shows that the impossible becomes possible when resources, will and science come together. Measles, on the other hand, reminds us that negligence can turn everything upside down.
«The resurgence of preventable diseases like measles is a wake-up call. We must protect progress through sustained investment in vaccines and trust in science.»
— Tedros Adhanom Ghebreyesus, Director-General of the WHO (2022)
Health, whether human or animal, is a fragile common good that must always be defended
References
-
Anderson, J., and J. A. McKay. 1994. “The Detection of Antibodies against Peste Des Petits Ruminants Virus in Cattle, Sheep and Goats and the Possible Implications to Rinderpest Control Programmes.” Epidemiology and Infection 112 (1): 225–31.
-
Bankamp, Bettina, Makoto Takeda, Yan Zhang, Wenbo Xu, and Paul A. Rota. 2011. “Genetic Characterization of Measles Vaccine Strains.” The Journal of Infectious Diseases 204 Suppl 1 (suppl_1): S533-48.
-
Brozio, Jan Piet, Jutta Kneisel, Stefanie Schaefer-Di Maida, Julian Laabs, Ingo Feeser, Artur Ribeiro, and Sebastian Schultrich. 2024. “Patterns of Socio-Economic Cultural Transformations in Neolithic and Bronze Age Societies in the Central Northern European Plain.” In Perspectives on Socio-Environmental Transformations in Ancient Europe, 105–42. Cham: Springer Nature Switzerland.
-
Butler, Declan. 2019. “Sequence and Destroy: The Quest to Eliminate the Last Stocks of Deadly Rinderpest Virus.” Nature 572 (7767): 18.
-
Clark, Gregory. 2014. “Ian Morris. The Measure of Civilization: How Social Development Decides the Fate of Nations.” The American Historical Review 119 (3): 824–26.
-
Düx, Ariane, Sebastian Lequime, Livia Victoria Patrono, Bram Vrancken, Sengül Boral, Jan F. Gogarten, Antonia Hilbig, et al. 2020. “Measles Virus and Rinderpest Virus Divergence Dated to the Sixth Century BCE.” Science (New York, N.Y.) 368 (6497): 1367–70.
-
Fulton, Benjamin O., David Sachs, Shannon M. Beaty, Sohui T. Won, Benhur Lee, Peter Palese, and Nicholas S. Heaton. 2015. “Mutational Analysis of Measles Virus Suggests Constraints on Antigenic Variation of the Glycoproteins.” Cell Reports 11 (9): 1331–38.
-
Huygelen, C. 1997. “The Immunization of Cattle against Rinderpest in Eighteenth-Century Europe.” Medical History 41 (2): 182–96.
-
Inoue, Hiroko, Alexis Álvarez, Eugene N. Anderson, Andrew Owen, Rebecca Álvarez, Kirk Lawrence, and Christopher Chase-Dunn. 2015. “Urban Scale Shifts since the Bronze Age: Upsweeps, Collapses, and Semiperipheral Development.” Social Science History 39 (2): 175–200.
-
Lazarus, Jeffrey V., Katarzyna Wyka, Trenton M. White, Camila A. Picchio, Lawrence O. Gostin, Heidi J. Larson, Kenneth Rabin, Scott C. Ratzan, Adeeba Kamarulzaman, and Ayman El-Mohandes. 2023. “A Survey of COVID-19 Vaccine Acceptance across 23 Countries in 2022.” Nature Medicine 29 (2): 366–75.
-
Mina, Michael J., Tomasz Kula, Yumei Leng, Mamie Li, Rory D. de Vries, Mikael Knip, Heli Siljander, et al. 2019. “Measles Virus Infection Diminishes Preexisting Antibodies That Offer Protection from Other Pathogens.” Science (New York, N.Y.) 366 (6465): 599–606.
-
Morens, David M. 2003. “Characterizing a ‘New’ Disease: Epizootic and Epidemic Anthrax, 1769-1780.” American Journal of Public Health 93 (6): 886–93.
-
Morens, David M., Edward C. Holmes, A. Sally Davis, and Jeffery K. Taubenberger. 2011. “Global Rinderpest Eradication: Lessons Learned and Why Humans Should Celebrate Too.” The Journal of Infectious Diseases 204 (4): 502–5.
-
Wilkinson, L. 1984. “Rinderpest and Mainstream Infectious Disease Concepts in the Eighteenth Century.” Medical History 28 (2): 129–50.
-
Zemella, Anne, Kerstin Beer, Franziska Ramm, Dana Wenzel, Ariane Düx, Kevin Merkel, Sebastien Calvignac-Spencer, et al. 2024. “Vaccine-Induced Neutralizing Antibodies Bind to the H Protein of a Historical Measles Virus.” International Journal of Medical Microbiology: IJMM 314 (151607): 151607.
Enjoy Reading This Article?
Here are some more articles you might like to read next: